In a letter to Union health minister, Association of Indian Medical Device Industry had said move would ‘increase risk of importing contaminated products & hurt their interests’.
The Narendra Modi government is reviewing an earlier order to allow import of some pre-owned medical devices in the country following strong objections from domestic manufacturers, ThePrint has learnt.
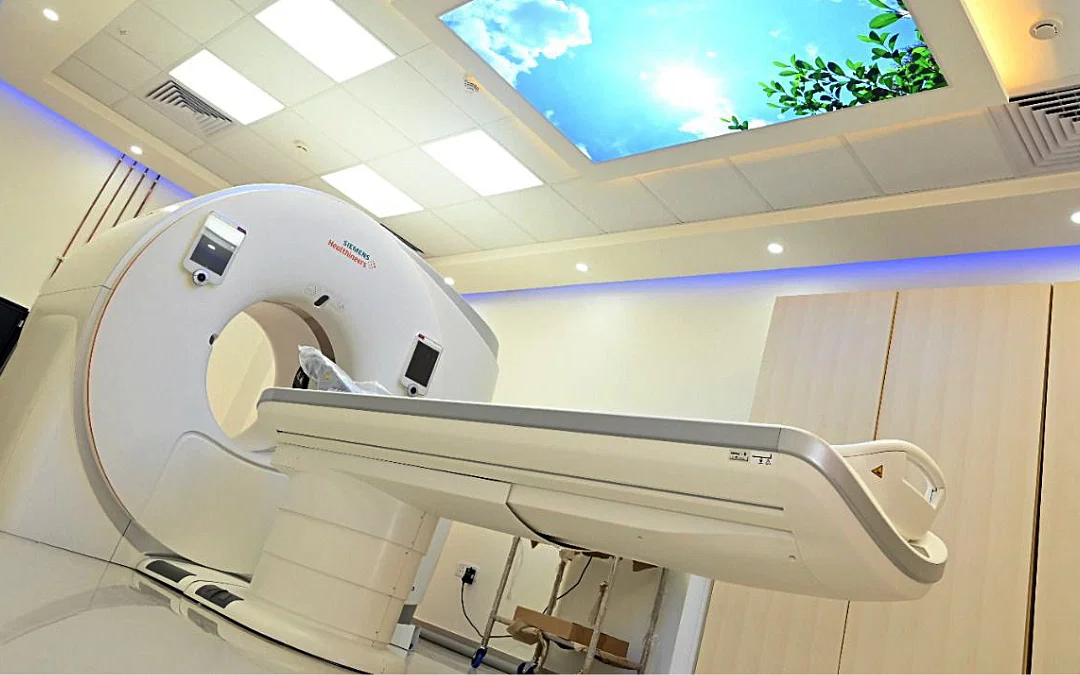
The Union Ministry of Environment, Forests and Climate Change had permitted the import of 50 high-end and high-value pre-owned medical equipment in an office memorandum (OM) issued on 19 June. These equipment included magnetic resonance imaging (MRI) and computerised tomography (CT) scan machines.
Some of the other used or pre-owned equipment now permitted for import in the country, according to the ministry’s order, include mammography machine, blood cell count analyser, high-end X-Ray machines, positron emission tomography-CT (PET-CT) scan machine and radiotherapy devices, among others.
The reason for allowing the import of these pre-owned medical devices was to make them affordable for small hospitals and healthcare establishments, especially in tier 2 and tier 3 cities, said government sources, adding that the decision was based on consultation with the Union Health Ministry. The OM was, however, issued by the Environment, Forests and Climate Change ministry as the import of used devices is also considered a potential environmental hazard.
In a letter written last week to Union Health Minister Mansukh Mandaviya by Association of Indian Medical Device Industry (AiMeD) coordinator Rajiv Nath, domestic device makers have raised their objections to the decision, claiming it will increase the risk of importing contaminated products (a reference to threat of environmental contamination), hurt their interests and not benefit patients in the long run. ThePrint has seen a copy of the letter.
Talking to ThePrint, Nath pointed out that the move serves as a big blow to the nascent medical device industry in India, which is trying to stand on its own feet. “Of the 50 used medical devices permitted for import now, 40 are already manufactured in India,” he stressed.
In a statement issued last week, AiMeD also accused the Federation of Indian Chambers of Commerce and Industry (FICCI) of “playing in the hands of the MNC lobby [sic]” and influencing the government’s decision.
AiMeD has alleged that the order by the Ministry of Environment, Forests and Climate Change was based on inputs given by the FICCI.
FICCI, however, defended the government order and told ThePrint in a written statement last week that the government decision to allow the import of pre-owned medical devices was based on the recommendations by an inter-ministerial committee to decide the list of high-end and high-value equipment not manufactured in the country under strict quality restrictions to be allowed for import to increase the access to healthcare to tier 2 and 3 cities”.
Meanwhile, a senior official in the health ministry told ThePrint that the matter has been referred to the director general of health services. A meeting with representatives of medical device manufacturers has also been called later this week, informed a second official.
ThePrint reached Union health secretary Sudhansh Pant over phone for comment but received no response till the time of publication of this report. The article will be updated once a response is received.
‘No benefit to patients or industry’
In its letter to Mandaviya, AiMeD has claimed that the import of pre-owned medical devices will neither help the industry, nor patients.
“The allowing of pre-owned medical equipment and re-manufacturing or re-furbishing of medical imaging systems and other medical electronic equipment will increase the risk of importing contaminated products [a reference to threat of environmental contamination] into the country,” the letter said.
“This will slow down investments in the sector, discourage domestic manufacturers and hurt patient interest in the long term,” it added.
The letter also highlighted that “We must understand the motives of Multinational Corporations (MNCs) who want to dump their obsolete and old equipment in India, as these used equipment have no market in their own countries. They are replacing them with new products and new technology”.
The letter also argued that pre-owned products are not allowed in countries like China, Vietnam, Thailand, Indonesia, Peru and Egypt, among others.
Speaking against the government decision, Nath argued if some hospitals do procure the equipment at cheaper rates, they will not pass the benefits to patients by making the services or cost of treatment more affordable. “In any case, all the new medical devices are allowed for import in India and as such there is no problem of access to these machines.”
ThePrint reached Girdhar J. Gyani, director general of the Association of Healthcare Providers of India — a network of private hospitals in the country — over phone for comments, but received no response. The article will be updated once a response is received.
According to government estimates, over 80 percent of new medical devices used in the country are imported, while the import cost of these machines stands at around Rs 60,000 crore annually.
‘A regressive step’
AiMeD has also alleged that the order by the Ministry of Environment, Forests and Climate Change was based on inputs given by the FICCI, which “was playing in the hands of the MNC lobby [sic]”.
“Nothing could have been worse than what FICCI Medical Devices Division is doing at the behest of MNCs lobby,” said the organisation in a statement last week.
“The decision to permit the import of pre-owned medical devices is in contravention to India’s National Medical Device Policy-2023 that seeks to make our country not only Atma Nirbhar (self-reliant) in medical devices, but also the global leader,” the statement said.
The association added that the order by the environment ministry is a regressive step that has created confusion among the investors who have been setting up manufacturing capacity in the last few years in response to the government’s call for self-reliance.
However, a FICCI spokesperson told ThePrint in a written statement that the government order was based on the recommendations by an inter-ministerial committee.
“The refurbished equipment being imported should be of very high quality providing precise diagnosis and treatment at costs which are affordable in India,” it said, highlighting one of the recommendations of the panel.
“FICCI is aligned with the Hon’ble Prime Minister and Government of India’s vision of Make in India and providing advanced and quality healthcare products and services available at affordable prices to the poorest of the poor population,” the FICCI statement added.
Meanwhile, the Medical Technology Association of India (MTaI), which represents research-based medical technology companies, has also said that the government order can increase accessibility of these capital-intensive equipment for Indian patients.
“Complex medical equipment like CT scans, MRI, Robotic-assisted surgical systems, X-Ray machines, endoscope, among others, cost multiple millions of dollars, which are often over the budgetary capability of smaller to mid-sized hospitals,” said MTaI chairman Pavan Choudary in a statement last week.
He added: “Pre-owned medical equipment, which are backed by adequate service guarantees and abide by the regulatory requirements can go a long way in solving this problem of access and affordability.”