The medical devices industry remains bitterly divided even as the Centre looks to regulate import of pre-owned products
Away from the glare of very public discussions on stents, a device used largely in the heart, another debate engulfs the medical devices industry in the country. And that involves allowing the import of refurbished or pre-owned medical equipment.
As the Centre explores the issue with an inter-ministerial committee looking into it, the discussion has divided the industry along the predictable lines of local and foreign parentage.
Local company representatives advocate an outright ban on the grounds that refurbished medical equipment pose safety and radiation concerns to patients and users, besides disincentivising local manufacturing.
But foreign companies based in India feel the concept is not fully understood and people are seeing ghosts where there aren’t any. In fact, there are price benefits to be got, they say, that could make refurbished products more affordable to clinics and hospitals in smaller cities.
The key differentiation is between refurbished products from genuine Original Equipment Manufacturers (OEM) and non-OEM imports by traders, explains Pavan Choudary, Director-General of the Medical Technology Association of India (MTal), a platform of foreign companies with investments in India.
Genuine refurbished goods involve rehauling a product with genuine spares for an additional run and the equipment come with safety and quality guarantees of an original product, he says. Importing of non-OEM products is done by traders, unsupported by guarantees and hence pose a safety threat to patients, he explains.
Of the 2,800 pre-owned equipment imported in the last two years, only 120 were OEM, says Choudary. About 95 per cent is sourced from non-OEM players and that is worrisome, say MTal representatives urging the Government to regulate and allow only those “revitalised” products that abide by safety rules.
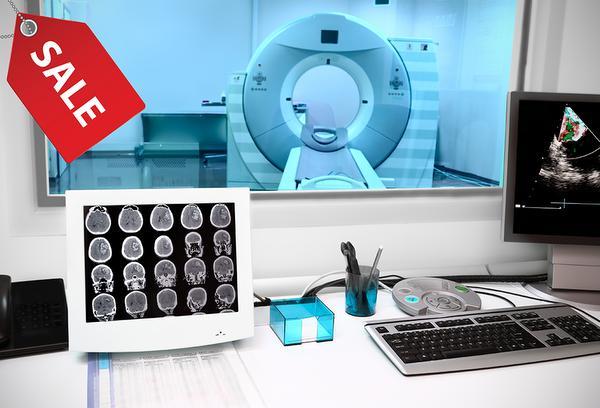
The Government needs to understand that refurbished medical equipment does not mean second-hand goods, says V Raja, Vice-Chairman and Managing Director of Philips India Limited. The import of a refurbished CT scanner, for example, would need AERB (Atomic Energy Regulatory Board) approvals for the product and the site, he says.
The present thinking is to allow refurbished products that are less than 10 years old. Refurbished products are usually priced about 35 per cent less than the original, he says, agreeing with industry estimates.
Dumping worries
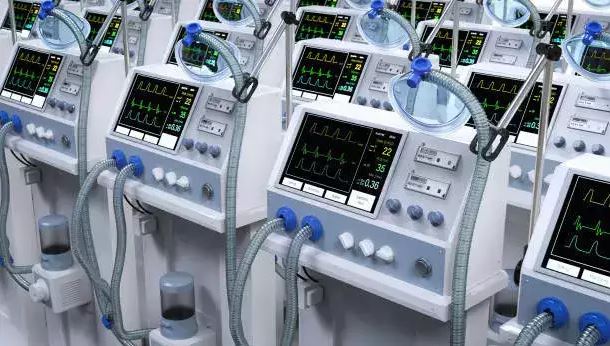
Addressing the fear of old medical equipment (CTs, MRI, Cathlab, PETCT) being dumped in India and other emerging markets, Choudary says the US, the UK, Singapore and Japan are open to such products, provided they abide by their rules. Over 60 per cent of all pre-owned products sold are supplied to the US, adds another official with a multinational healthcare company. The biggest names in the med-tech space including Philips, GE Healthcare and Siemens operate in this space.
The products come with a clear history of maintenance and manage to get another seven to 10 years of life, within stipulated limits on radiation etc. And at reduced prices, it becomes affordable to hospitals and clinics in Tier 2 and 3 cities, he says. Besides, products that are imported are those that are not being made by local manufacturers, he adds, responding to queries on whether these imports kill local manufacturing.
‘No price benefit’
Domestic med-tech manufacturers though don’t agree with their foreign counterparts. Insisting that refurbished products are unsafe for patients. Rajiv Nath, who represents the Association of Indian Medical Device Manufacturers (AIMED). says there is no transparency around the recalibration of these products, there are no support services and, most importantly, the price benefits from pre-owned products are not passedw on to the patient.
The situation could lead to doubtful “branded” refurbished products circulating in the market, brought in by traders and not manufacturers, he cautions.
With industry this divided, the onus now shifts back to the Government to navigate a middle ground and provide a safe pathway that would be in the interest of patients.
Publication: The Hindu Businessline (Clip attached)
Journalist: P T Jyoti Dutta